touchCONGRESS Advances in immunotherapies and targeted treatments for patients with bladder cancer: An update from ESMO 2022
Watch this two-part activity exploring recent advances in immunotherapies and targeted treatments for patients with bladder cancer. Filmed following the ESMO 2022 Congress.
Part 1: Watch urological cancer expert Prof. Axel Merseburger review key data from the ESMO Congress 2022 Watch Now
Part 2: Choose from leading experts who discuss what these data may mean for global and regional practice Select An Interview
Introduction
Latest efficacy and safety data for immunotherapies and targeted treatments for bladder cancer
Future directions in the use of immunotherapy and targeted therapies for the treatment of bladder cancer
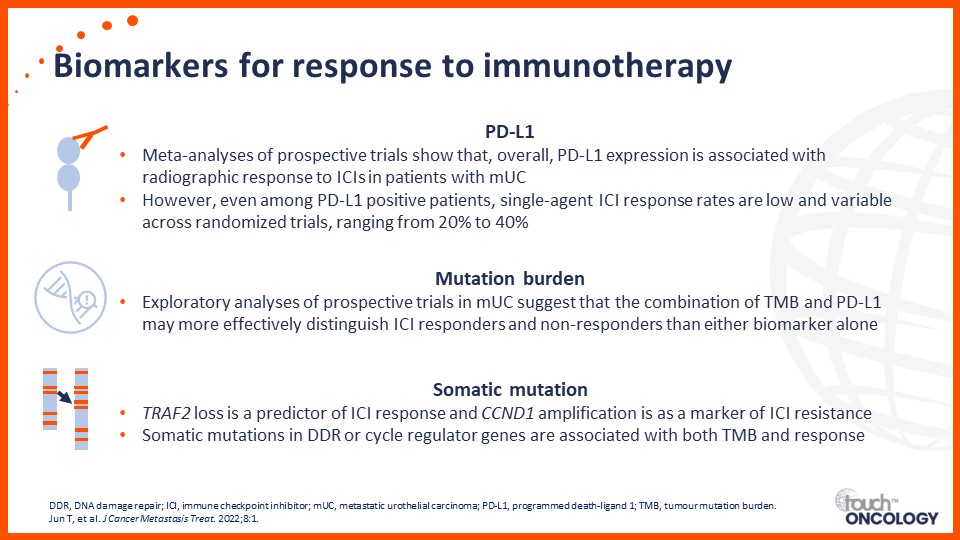
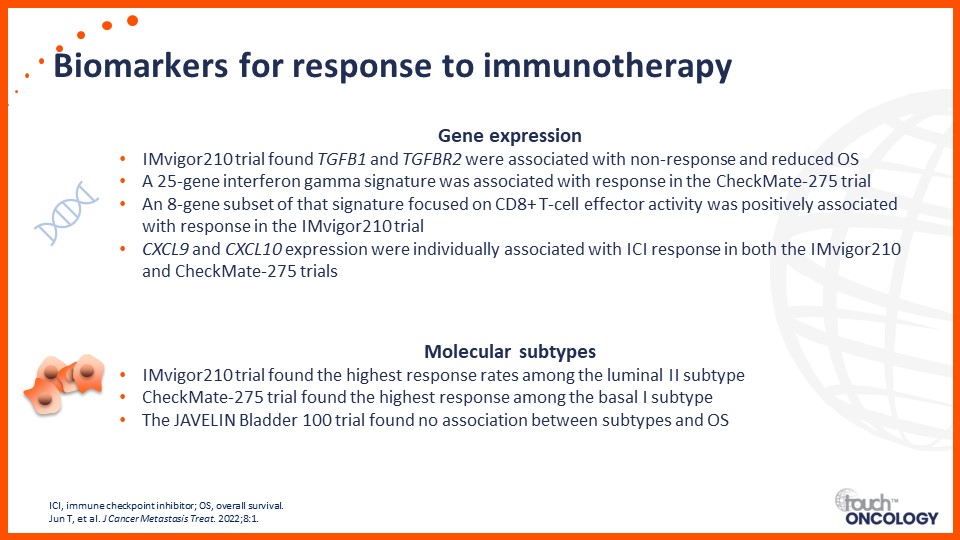
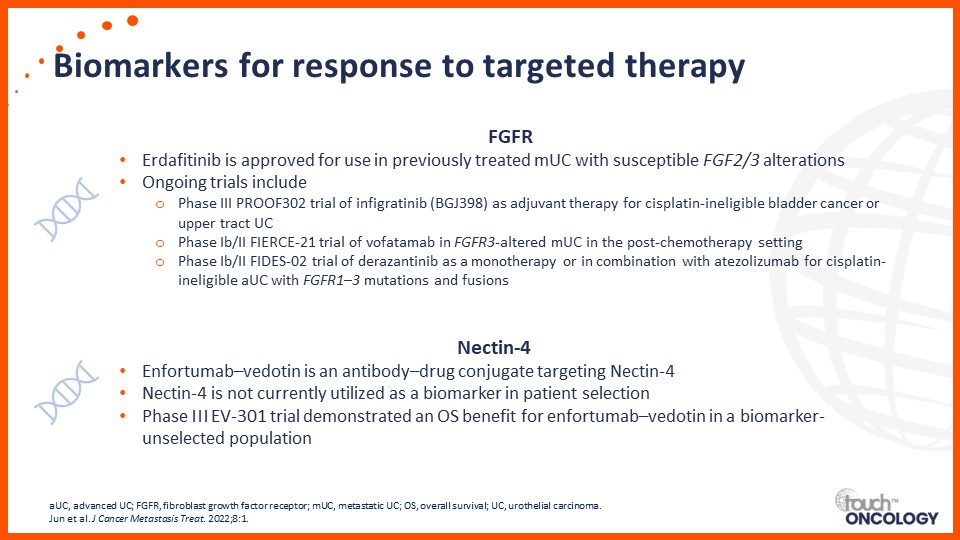
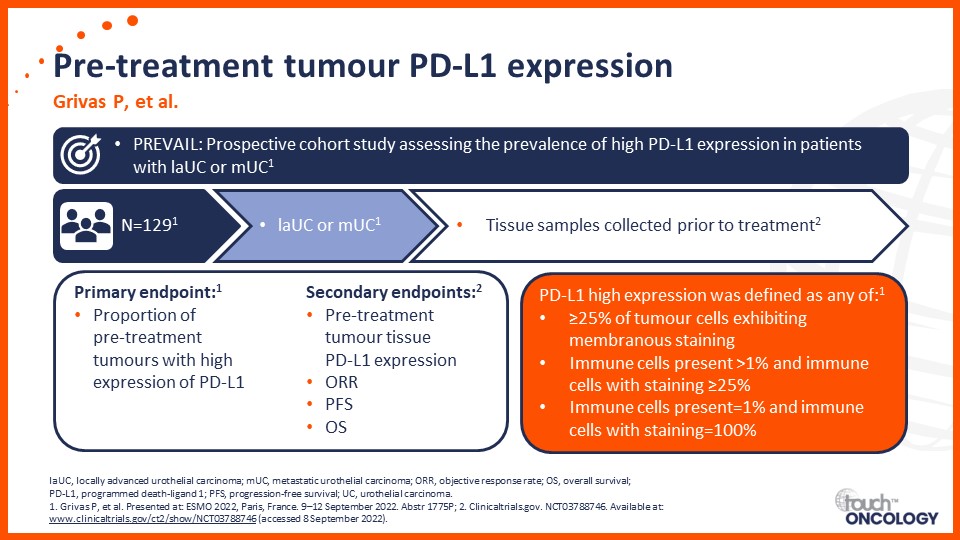
Recent data on biomarkers and prognostic factors for immunotherapy and targeted treatment outcomes in patients with bladder cancer
Overview
Watch Prof. Axel Merseburger summarize and share his interpretation of the latest data from the ESMO Congress 2022 on immunotherapies and targeted treatments for bladder cancer. During the presentation he considers:
- Latest efficacy and safety data for immunotherapies and targeted treatments for bladder cancer
- Future directions in the use of immunotherapy and targeted therapies for the treatment of bladder cancer
- Recent data on biomarkers and prognostic factors for immunotherapy and targeted treatment outcomes in patients with bladder cancer
Axel Merseburger is professor of urology and chairman of the Department of Urology at University Hospital Schleswig-Holstein, Campus Lübeck, Germany. read more
Prof. Merseburger’s research activity encompasses both molecular and clinical aspects of uro-oncology, with specific interest in biomarkers and prognostic factors for prostate cancer, renal cell carcinoma and transitional cell carcinoma.
Prof. Merseburger is a member of various uro-oncology organisations, he is the chairman elect for the European Scholarship Program (ESUP) of the European Association of Urology (EAU) and was the chairman of the EAU Guideline Group for Lasers and Technologies. He acts as reviewer and editorial board member for several urology and oncology indexed journals, and is associate editor of the World Journal of Urology and editor-in-chief of the European Journal of Haematology and Oncology and Aktuelle Urologie.
He has authored and co-authored more than 300 peer-reviewed articles, and he is the principal investigator in multiple phase II and III clinical trials within the field of urologic oncology.
Prof. Axel Merseburger discloses Advisory board or panel fees from Astellas, Bayer, Ipsen, Janssen and Roche. Consultancy fees from Astellas, Bayer, Ipsen, Janssen and Roche. Speaker’s bureau fees from Astellas, Bayer, Ipsen, Janssen and Roche.
Dr McGregor considers the latest data from ESMO Congress 2022 on existing and emerging treatments for bladder cancer, their role in the improvement of patient outcomes and their impact on daily practice.
Prof. Axel Merseburger considers the latest data from ESMO Congress 2022 on existing and emerging treatments for bladder cancer, their role in the improvement of patient outcomes and their impact on daily practice.
Prof. Andrea Necchi considers the latest data from ESMO Congress 2022 on existing and emerging treatments for bladder cancer, their role in the improvement of patient outcomes and their impact on daily practice.
Please Select A Video:
Overview & Learning Objectives
Overview
Stay up to date with the latest data on existing and emerging treatments for bladder cancer and how clinical practice is changing to improve patient outcomes in this two-part activity. This activity was filmed during the ESMO Congress 2022 (Paris, 9–12 September 2022).
This activity is jointly provided by USF Health and touchIME. read more
Target Audience
This activity has been designed to meet the educational needs of oncologists, including genitourinary cancer sub-subspecialists and urologists involved in the management of patients with bladder cancer.
Disclosures
USF Health adheres to the Standards for Integrity and Independence in Accredited Continuing Education. All individuals in a position to influence content have disclosed to USF Health any financial relationship with an ineligible organization. USF Health has reviewed and mitigated all relevant financial relationships related to the content of the activity. The relevant financial relationships are listed below. All individuals not listed have no relevant financial relationships.
Faculty
Dr Bradley McGregor discloses: Advisory board or panel fees from Astellas, Bristol Myers Squibb (BMS), Calithera Biosciences, Dendreon, Eisai, EMD Serono, Exelixis, Merck KGaA, Pfizer and Seattle Genetics. Consultancy fees from Janssen Oncology. Grants/research support from Astellas, Bristol Myers Squibb (BMS), Calithera Biosciences, EMD Serono, Exelixis, Pfizer and Seattle Genetics.
Prof. Axel Merseburger discloses: Advisory board or panel fees from Astellas, Bayer, Ipsen, Janssen and Roche. Consultancy fees from Astellas, Bayer, Ipsen, Janssen and Roche. Speaker’s bureau fees from Astellas, Bayer, Ipsen, Janssen and Roche.
Prof. Andrea Necchi discloses: Advisory board or panel fees from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb (BMS), Catalym, Gilead, Incyte, Janssen, Merck, Pfizer and Roche. Consultancy fees from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb (BMS), Catalym, Gilead, Incyte, Janssen, Merck, Pfizer and Roche. Grants research support from AstraZeneca, Gilead and Merck.
Content reviewer
Lucas Wiegand, MD has no relevant financial relationships to disclose.
Touch Medical Director
Adriano Boasso has no financial interests/relationships or affiliations in relation to this activity.
USF Health Office of Continuing Professional Development and touchIME staff have no financial interests/relationships or affiliations in relation to this activity.
Requirements for Successful Completion
In order to receive credit for this activity, participants must review the content and complete the post-test and evaluation form. Statements of credit are awarded upon successful completion of the post-test and evaluation form.
If you have questions regarding credit please contact cpdsupport@usf.edu.
Accreditations
Physicians
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through a joint providership of USF Health and touchIME. USF Health is accredited by the ACCME to provide continuing medical education for physicians.
USF Health designates this enduring material for a maximum of 1.0 AMA PRA Category 1 CreditTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
The European Union of Medical Specialists (UEMS) – European Accreditation Council for Continuing Medical Education (EACCME) has an agreement of mutual recognition of continuing medical education (CME) credit with the American Medical Association (AMA). European physicians interested in converting AMA PRA Category 1 CreditTM into European CME credit (ECMEC) should contact the UEMS (www.uems.eu).
Advanced Practice Providers
Physician Assistants may claim a maximum of 1.0 Category 1 credits for completing this activity. NCCPA accepts AMA PRA Category 1 CreditTM from organizations accredited by ACCME or a recognized state medical society.
The AANPCP accepts certificates of participation for educational activities approved for AMA PRA Category 1 CreditTM by ACCME-accredited providers. APRNs who participate will receive a certificate of completion commensurate with the extent of their participation.
Date of original release: 3 October 2022. Date credits expire: 3 October 2023.
If you have any questions regarding credit please contact cpdsupport@usf.edu.
Learning Objectives
After watching this activity, participants should be better able to:
- Recall the latest efficacy data for new and emerging targeted treatments for patients with bladder cancer
- Describe the safety profile of new and emerging targeted treatments for patients with bladder cancer
- Discuss how the latest data on targeted treatments may affect the current and future management of patients with bladder cancer

Register to touchONCOLOGY for FREE
- Peer-reviewed journals and expert opinions
- Interactive CME and e-learning modules
- Video conference highlights